Q&A: Dr Olivia Morris Hanon on Brain Cancer Research
Dr Olivia Morris Hanon shares insights into her research uncovering how brain cancer cells communicate and survive.

Inside the Research Driving Hope for Brain Cancer Patients
Brain cancer remains one of the most complex and challenging diseases to treat, but researchers in South Australia are leading the charge toward new discoveries and better outcomes for patients. We spoke with Dr Olivia Morris Hanon , whose current work focuses on understanding the intricate relationship between brain cells and tumour cells in glioblastoma, the most aggressive form of brain cancer.

What inspired you to focus your research on brain cancer?
I started working with glioblastoma during my bachelor’s degree and became engrossed with this complex and contradictory tumour. An oncologist I collaborated with pointed out that it was the most heterogeneous tumour he had worked with regarding its histology, behaviour, and markers, but it was also one of the most homogeneous in its prognosis and outcome. There is still much we don’t know about this tumour, and until we fill that knowledge gap, the development of new and effective treatments will remain slow and challenging.
Can you describe the main goal of your current project and how it could eventually benefit patients?
Tumour cells on the edge of the tumour can integrate into the brain’s normal neural networks, leading to tumour survival and worse neurological symptoms for the patient. These tumour cells appear to blend in with healthy neurons by using the same communication signals the brain relies on, such as GABAergic neurotransmission. By studying how these interactions occur, we aim to uncover the mechanisms that enable glioblastoma cells to survive and resist standard treatments such as chemotherapy and radiation. Using advanced organoid models that mimic the human brain, we can observe how tumour and neuronal cells communicate in real time and test ways to disrupt these connections. In the long term, this research could lead to new treatment strategies that make brain tumour cells more vulnerable to existing therapies. Instead of only trying to kill tumour cells directly, this approach targets the “conversations” between cancer and brain cells that help the tumour persist. Ultimately, this could improve both survival and neurological outcomes for patients with glioblastoma by preventing tumour regrowth while protecting healthy brain function.
In very basic terms, what methods are you using in this project?
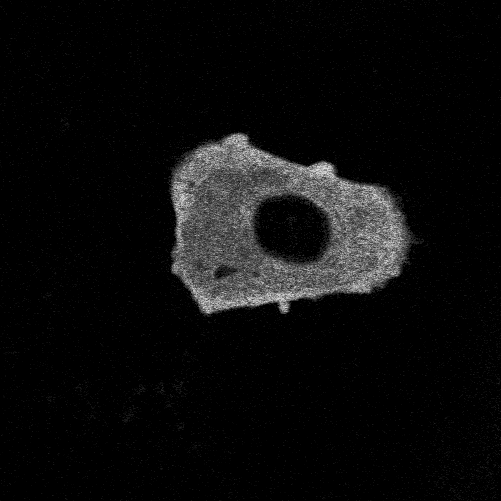
To study glioblastoma, we use glioblastoma organoids: three-dimensional spheres created from patient tumour tissue that contain not only the malignant cells but also immune cells, non-tumour glial cells, and other components of the tumour environment. This model is better at predicting how a tumour will respond to treatments compared to traditional cell cultures.
To study the interactions we are engineering a better model in which these organoids also contain neurons so that we can study how the interactions between tumour cells and neurons promote tumour progression and so that we may identify ways to intervene and prevent tumour cells from integrating into brain networks.
Are you working with other labs or institutions?
Our team collaborates with many research groups in Australia and overseas, which allows us to expand our skill sets and access new technologies. For my project, we are establishing a collaboration with Dr. Ernst Wolvetang and Dr. Selin Pars at the University of Queensland, neuroscientists who specialise in epilepsy research. Our hope is that by combining neuroscience and cancer biology, we can effectively study the interactions between the brain and tumour cells and target the pathways that allow glioblastoma to connect and thrive within neural networks.

You attended an overseas research trip this year — what skills or insights did you gain and how will this impact SA research?
During my visit to the University of Virginia, funded by the NRF, I received specialised training in FRET/FLIM imaging, an advanced form of microscopy that uses light to study how molecules behave inside living cells. This technique allows scientists to see when two proteins interact, how a cell’s metabolism changes, and how signals, such as calcium, move between cells. I was particularly interested in learning how to design light-based sensors that can detect calcium levels inside cells as calcium is a key signal the brain uses for communication, and by tracking it we can learn when and how cells form connections with each other. In brain tumours such as glioblastoma, these same signals can be hijacked by cancer cells to grow and spread, so being able to measure them is extremely valuable. At the Centre for Cancer Biology, we have access to world-class microscopes that are well suited for these techniques. By receiving this training, I can now help our group make full use of this equipment to advance our research, not only for my own project but also to support other researchers in our team.

What do you think are the biggest hurdles in translating preclinical findings into real-world therapies?
There are many hurdles in translating preclinical findings into clinical therapies, and they are particularly evident in glioblastoma research, where treatment options have changed little for decades. In the past, much of the research has relied on long-established cell lines and animal models that do not fully capture the complexity of human brain tumours, especially the diverse mix of cancer, immune, and neuronal cells that make up the tumour environment. These differences make it difficult to predict how a treatment will perform in patients. The development of more advanced laboratory models, such as organoids that better reproduce the composition and behaviour of human tumours, is a crucial step forward. By creating models that better represent the tumour landscape, we can increase the chances that promising discoveries in the lab will successfully translate into effective therapies for patients.
What message would you share with the public about the importance of supporting brain cancer research?
Over my years in research, I have seen how many tumours once thought incurable have become treatable thanks to steady, persistent scientific progress. Every breakthrough begins with small, often unseen steps, experiments that build on one another until they transform outcomes for patients and families. Brain cancer remains one of the most challenging diseases to treat, but we are now beginning to understand it in completely new ways. Advances in imaging, genetics, and stem cell research are revealing how brain tumours grow, adapt, and resist therapy. Each discovery brings us closer to treatments that not only extend life but also preserve the person, their thoughts, their memories, and their connections with others. Supporting brain cancer research means investing in hope. Hope grounded in science, and the belief that no diagnosis should come without options. With continued support, we can move from understanding to cures, turning what once seemed impossible into tomorrow’s new standard of care.

Dr Olivia Morris Hanon | Centre for Cancer Biology